Carbon Dioxide Contained in a Piston Cylinder Arrangement
Carbon dioxide C O 2 CO_2 C O 2 contained in a pistoncylinder arrangement initially at 6 bar and 400 K undergoes an expansion to a final temperature of 298 K during which the pressurevolume relationship is p V 12 p V12 p V 12 constant. Mass is added at such a rate that the gas compresses according to the relation P Y 12 constant to a final temperature of 350 F.
Solved Carbon Dioxide Co2 Contained In A Piston Cylinder Chegg Com
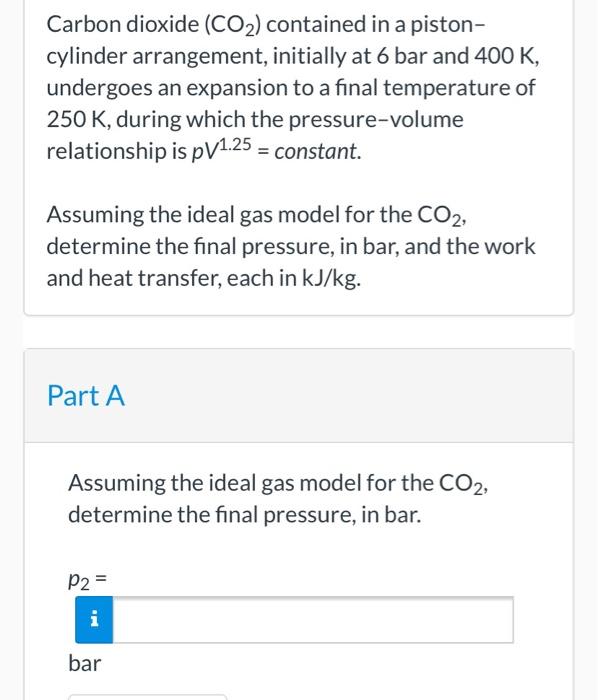
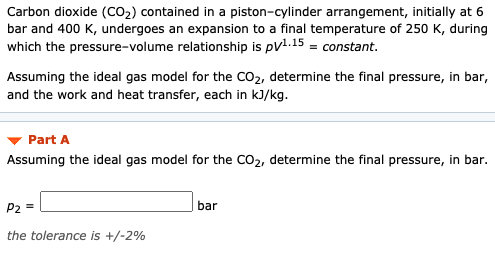
Carbon dioxide CO 2 contained in a pistoncylinder arrangement initially at 6 bar and 400 K undergoes an expansion to a final temperature of 250 K during which the pressurevolume relationship is pV 11 constant.

. The device has a mass of 4 kg. During the process the pressure and volume are related by P a V 2 where a 8 k P a m 6. This problem has been solved.
Carbon dioxide CO2 contained in a pistoncylinder arrangement initially at 6 bar and 400 K undergoes an expansion to a final temperature of 280 K during which the pressurevolume relationship is pV11 constant. Carbon dioxide CO2 contained in a piston-cylinder arrangement initially at 6 bar and 400 K undergoes an expansion to a final temperature of 298 K during which the pressure-volume relationship is pV12 constant. A pistoncylinder contains carbon dioxide at 300 kPa 100C with a volume of 02 m3.
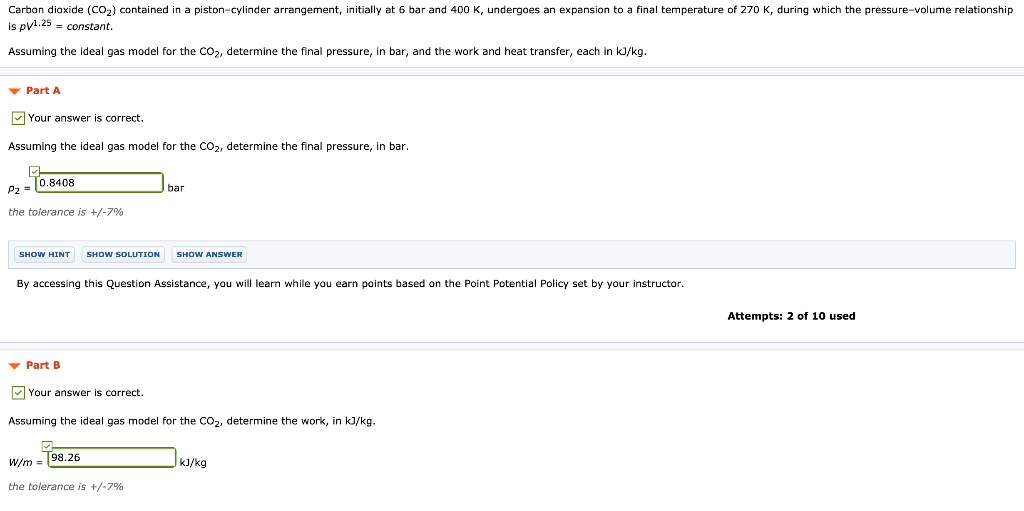
Carbon dioxide CO2 contained in a pistoncylinder arrangement initially at 6 bar and 400 K undergoes an expansion to a final temperature of 298 K during which the pressure volume relationship is pV12 constant. Determine the work done during the process. Assuming the ideal gas model for the CO 2 determine heat transfer in kJkg.
1 MPa is contained in a piston-cylinder arrangement with an initial volume of 01 m3. Answer View Answer Discussion You must be signed in to discuss. The initial pressure and temperature of the gas are 4567 kPa and 123 deg C respectively.
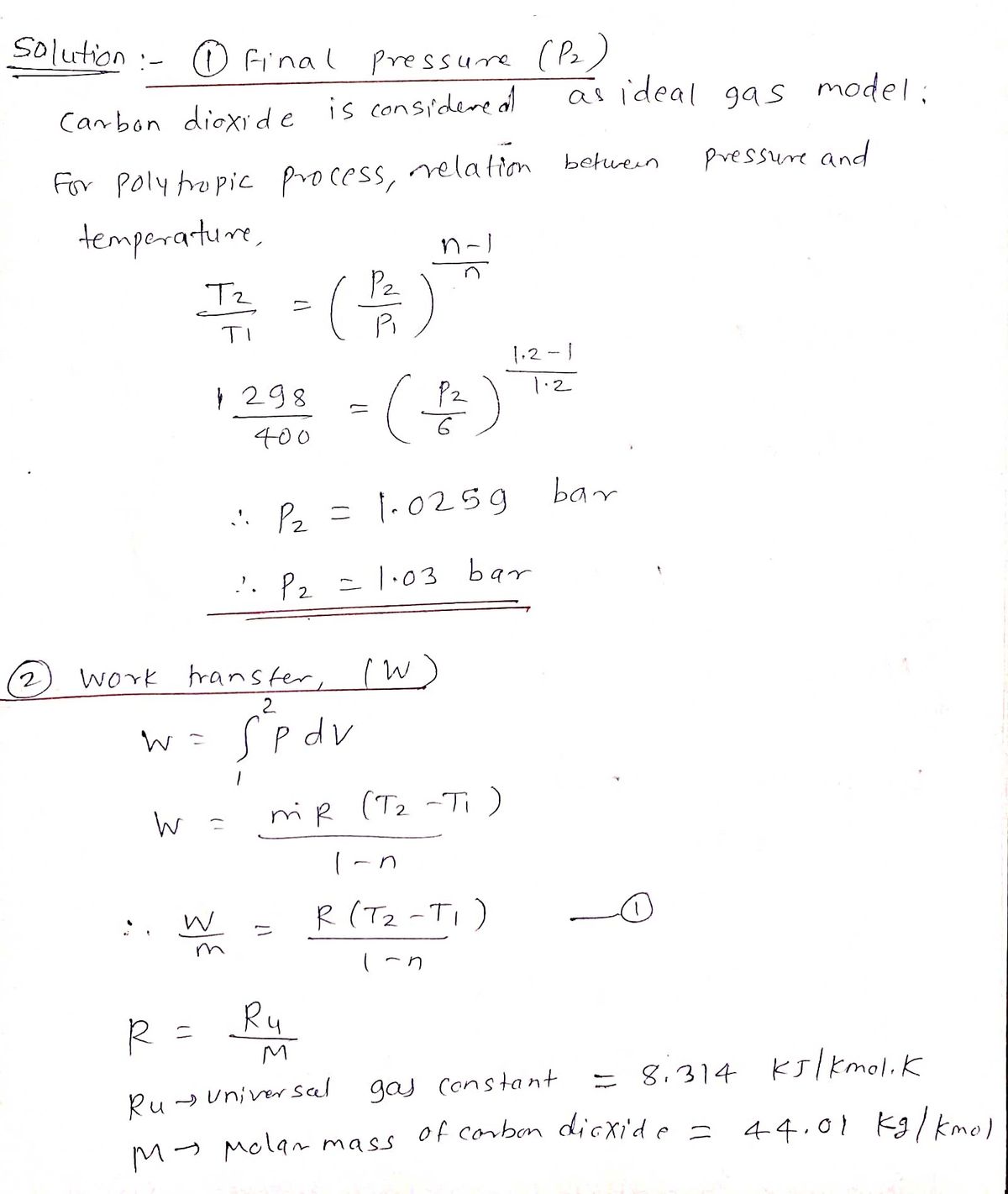
Calculate the work done on the carbon dioxide during this process. From the figure the heat flows from cold water to h. Carbon dioxide CO2 contained in a piston-cylinder arrangement initially at 6 bar and 400 K undergoes an expansion to a final temperature of 260 K during which the pressure-volume relationship is pV125 constant.
The pistoncylinder shown in Fig. Draw the figure of parallel flow arrangement. During the process the pressure and volume are related by P aV-2 where a 8 kPam6.
P448 contains carbon dioxide at 50 l b f i n 2 200 F with a volume of 5 f t 3. Carbon dioxide CO2 contained in a pistoncylinder arrangement initially at 6 bar and 400 K undergoes an expansion to a final temperature of 298 K during which the pressurevolume relationship is pV12 constant. Determine the work for this process.
Assuming the ideal gas model for the CO2 determine the final pressure in bar and the work and heat transfer each in kJkg. Carbon dioxide CO2 contained in a piston-cylinder arrangement initially at 6 bar and 400 K undergoes an expansion to a final temperature of 298 K during which the pressure-volume relationship is pV12constant. Carbon dioxide CO 2 contained in a pistoncylinder arrangement initially at 6 bar and 400 K undergoes an expansion to a final temperature of 280 K during which the pressurevolume relationship is pV12 constant.
A piston-cylinder contains carbon dioxide at 2MPa with V50 L. Assuming the ideal gas model for the CO2 determine the final pressure in bar and the work and heat transfer each in kJkg. Assuming the ideal gas model for the CO2 determine the final pressure in bar and the work and heat transfer each in kJkg.
Carbon dioxide contained in a piston-cylinder arrangement initially at 6 bar and 400K undergoes an expansion to a final temperature of 298 k during which the pressure-volume relationship if pV12 constant. A piston cylinder arrangement contains 011 kg of Nitrogen and recieves 1178 kJ of heat isothermally. R-12 in a piston-cylinder arrangement is initially at 50C x 1.
If temperature is maintained find the final pressure in kPa. Carbon dioxide CO2 contained in a pistoncylinder arrangement initially at 6 bar and 400 K undergoes an expansion to a final temperature of 298 K during which the pressurevolume relationship is pV12 constant. Assuming the ideal gas model for the CO2 determine the final pressure in bar and the work and heat transfer each in kJkg.
Assuming the ideal gas model for the CO2 determine the final pressure in bar and the work and heat transfer each in kJkg. Carbon dioxide CO2 contained in a pistoncylinder arrangement initially at 6 bar and 400 K undergoes an expansion to a final temperature of 280 K during which the pressurevolume relationship is pV125 constant. Determine the specific gravity of carbon dioxide gas R 018896 kJkgm K.
Carbon dioxide contained in a piston-cylinder arrangement initially at 6 bar and 400K undergoes an expansion to a final temperature of 298 k during which the pressure-volume relationship if pV12 constant. Assuming the ideal gas model for the CO 2 determine the final pressure in bar and the work and heat transfer each in kJkg. Carbon dioxide contained in a piston-cylinder device is compressed from 03 to 01 m 3.
Assuming the ideal gas model for the CO2 determine the final pressure in bar and the work and heat transfer each in kJkg. Assuming the ideal gas model for the CO2 determine the final pressure in bar and the work and heat transfer each in kJkg. Assuming the ideal gas model for the CO2 determine the final pressure in bar and the work and heat transfer each in kJkg.
Carbon dioxide contained in a piston-cylinder device is compressed from 03 to 01 m3. Calculate the work done on the carbon dioxide during this process. Assuming the ideal gas model for the CO2 determine the final pressure in bar and the work and heat transfer each in kJkg.
Assuming the gas to be an ideal gas determine the final pressure kPa the work done and the heat transfer each in kJ. Answer 52597 B t u R View Answer Discussion. A 232 kJkg.
It is then expanded in a process so that P Cv1 to a pressure of 100 kPa. A piston-cylinder arrangement contains Carbon dioxide CO2 initially at 66 kPa and 400 K undergoes an expansion process with pressure-volume relationship of PV 12 Costantto a final temperature of 298 K. Assuming the ideal gas model for the CO2 determine the heat transfer in kJkg.
Carbon dioxide CO2 contained in a pistoncylinder arrangement initially at 6 bar and 400 K undergoes an expansion to a final temperature of 300 K during which the pressurevolume relationship is pV125 constant. It is then slowly expanded according to the relation PV constant until a final pressure of 100 kPa is attained. Weights are added at such a rate that the gas compresses according to the.

Solved Carbon Dioxide Co2 Contained In A Piston Cylinder Chegg Com
Answered Carbon Dioxide Contained In A Bartleby
Solved Carbon Dioxide Co2 Contained In A Piston Cylinder Chegg Com
Solved Carbon Dioxide Co2 Contained In A Piston Cylinder Chegg Com
0 Response to "Carbon Dioxide Contained in a Piston Cylinder Arrangement"
Post a Comment